Learn how to write a standard SOP for disposal of rejected materials (raw and finished products) in the pharmaceutical industry.
1. PURPOSE
The purpose of this procedure is to establish a system for the destruction and disposal of rejected, damaged or expired raw materials and finished products.
2. SCOPE
This procedure applies to all rejected and expired general raw materials and finished products that have been approved for destruction in factory premises of this pharmaceutical.
3. ASSOCIATED DOCUMENTS
N/A
4. PRECAUTIONS
4.1. Use protective measures such as eye protector, protective clothing, mask and gloves during destruction the materials or products.
4.2. Ensure that only rejected materials are destructed.
5. RESPONSIBILITIES
5.1. Administration department is responsible for destruction the rejected products of materials.
5.2. Warehouse personnel are responsible for organizing the destruction of materials and finished
products in warehouse.
5.3. Quality Control personnel are responsible for destruction of expired raw materials in retention sample
room.
5.4. Quality Assurance personnel are responsible for destruction of expired finished products in retention
sample room.
5.5 Quality Assurance personnel are also responsible for monitoring, supervising and ensuring that the
materials or products are destroyed.
5.6 Engineering personnel are responsible for operate the incineration.
6. ACCOUNTABILITY
Head of the Quality Assurance Department
7. PROCEDURE
7.1 Form a materials / product disposal committee.
7.2 Ensure that Managing Director has approved the destruction of expired raw materials and finished products.
7.3 The detected raw materials and finished products will be destroyed for any of the following reasons:
7.4 Identify the destroyable items and receive any other rejected materials sent by QA Department for disposal.
7.5 Arrange to send the material to salvage area in case the material is disposable as scrap.
7.6 Arrange to dispose the rejected material or products as scrap if it is certified as scrap by QA department.
7.7 Raw materials:
7.7.1 Collect the raw materials from retention sample which are destroyable. Take necessary
precaution. Segregate the water soluble materials in a plastic bucket and soluble in water. When the materials are completely soluble then dilute with water. Discard the soluble materials in sink. Collect all water insoluble materials and transfer it incineration room for incinerate the materials.
7.7.2 Collect the raw materials from ware house which are expired. Segregate the water soluble
materials in a 50 kg plastic drum and dissolve in water. When the materials are completely soluble then dilute with water. Discard the soluble materials in ETP drain.
7.7.3 Keep the record in a log sheet
7.8 In-process or Finished Products:
7.8.1 Identify the destroyable In-process or finished products from retention sample room or sent by the
sales & distribution.
7.8.2 Collect all tablets and capsules in a segregate area and defoiling the products. Transfer
tablets and capsules individually in a plastic drum. Ass sufficient hot water in the drum and stir to dissolve. After completely dissolve the products ass with water . Discard the soluble products in ETP drain. Collect all foils in a master carton. Inner carton and insert put in another master carton. Cut the inner carton and inserts. Transfer all primary & secondary materials for scrap sale under administration.
7.8.3 Collect all PFS segregate the bottle from the inner carton. Open the cap from the bottle and
transfer the powder from the bottles in a plastic drum. Add sufficient hot water in the drum and stir to dissolve. After completely dissolve the products add with huge amount of water. Discard the soluble products in ETP drain. All bottles are washing with potable water and transfer to scrap sale. Transfer all primary & secondary materials for scrap sale under administration.
7.8.4 Keep a record for the materials/ products destroyed in rejected materials disposal form and keep it
in a destruction disposal files.
7.8.5 Organize disposal as scrap with supervision by security and keep a record of such material
disposed as scrap noting the quantity of material disposed and retain the record in disposal file.
7.8.5 Prepare written instruction in case of abnormal situation taking technical assistance for recovery
or disposal of raw materials/finished products, based on their characteristics and get the instruction approved by head of QA before undertaking the destruction work.
7.8.6 Write instruction for washing and cleaning of bottles and get it approved by head of QA if the
bottles/ containers are re-useable.
7.8.7 Arrange destruction of stock items in the factory premises in presences of the disposal Committee
and all those persons will sign on the write off proposal after destruction.
7.8.8 Record in a log sheet.
Read Also: SOP for Handling and Usage of Hazardous Chemicals
8. ABBREVIATIONS
8.1 SOP: Standard Operating Procedure
8.2 ETP: Effluent Treatment Plant
8.3 QA: Quality Assurance
8.4 QC: Quality Control
9. ANNEXURES
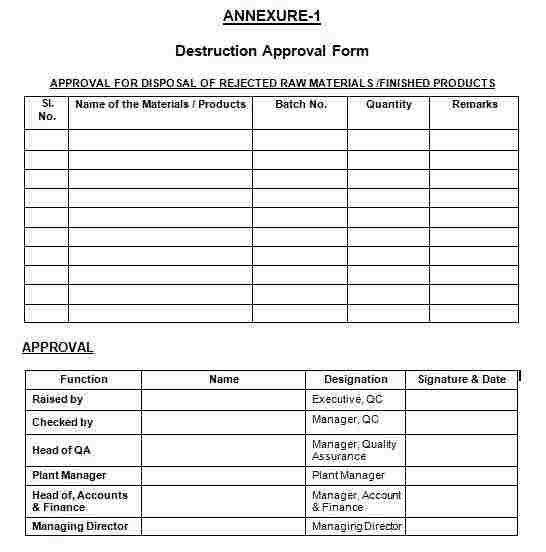
9.1 Annexure-1: Destruction Approval Form
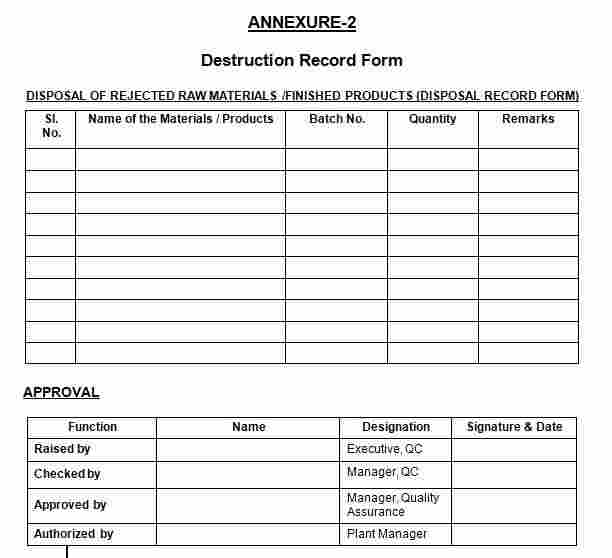
9.2 Annexure-2: Destruction Record Form

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



