CAPA stands for Corrective Action and Preventive Action. It is a systematic process used in the pharmaceutical industry to identify, correct, and prevent issues that can affect product quality, safety, or regulatory compliance. Corrective Action and Preventive Action is a key component of a quality management system (QMS) and is mandated by regulatory authorities such as the FDA, EMA, and WHO to ensure continuous improvement and product safety.
Table of Contents
The key elements of Corrective Action and Preventive Action
1. Corrective Action
Corrective Action refers to actions taken to eliminate the root cause of a detected non-conformance, deviation, or other quality-related issue to prevent its recurrence.
Steps in Corrective Action:
i) Problem Identification: Clearly define and document the issue, such as a deviation, defect, or non-compliance. Examples include product failures, out-of-specification results, or audit findings.
ii) Root Cause Analysis: Use tools like the 5 Whys, Fishbone Diagrams, or Failure Mode and Effects Analysis (FMEA) to identify the underlying cause of the problem, not just the symptoms.
iii) Implementation of Corrective Action: Develop and implement a plan to correct the root cause of the issue. This could involve revising processes, retraining employees, updating standard operating procedures (SOPs), or replacing defective equipment.
iv) Verification of Effectiveness: After implementing the corrective action, monitor the process or system to ensure the issue has been resolved and does not recur.
Goal:
To correct the problem and prevent its recurrence by addressing the root cause.
2. Preventive Action
Preventive Action involves proactive steps taken to identify potential issues before they occur and to eliminate the causes to prevent non-conformances from happening in the future.
Steps in Preventive Actions:
i) Risk Identification: Anticipate potential risks or issues through audits, risk assessments, process reviews, and trend analysis (e.g., out-of-trend results, increasing deviation frequencies).
ii) Root Cause Identification: Similar to corrective action, perform a root cause analysis to understand what might cause the potential problem.
iii) Preventive Measures Implementation: Implement actions to prevent the occurrence of the identified risks. This could include revising processes, conducting additional training, improving maintenance, or enhancing supplier quality management.
iv) Monitoring and Verification: Continuously monitor the system to ensure the preventive measures are effective and that no issues arise.
Goal:
To prevent potential issues from occurring by proactively identifying risks and eliminating the causes before they lead to problems.
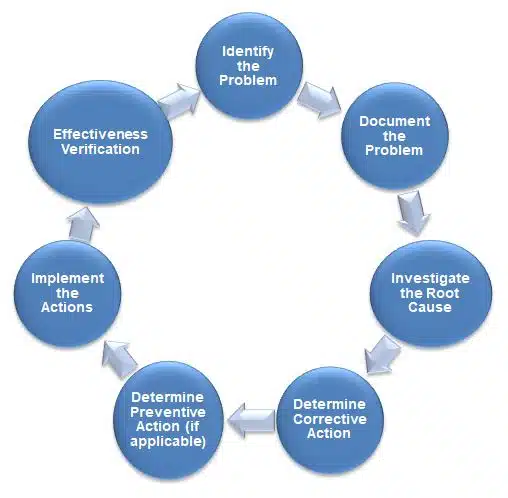
Corrective Action and Preventive Action Workflow
1) Identify the Problem
Non-conformance or potential issue is identified via deviation reports, customer complaints, audits, product testing, or trend analysis.
2) Document the Problem
The problem is documented in a CAPA report to ensure traceability and accountability.
3) Investigate the Root Cause
A detailed root cause analysis is conducted to find the underlying cause of the problem. This step is crucial to ensure the corrective action addresses the real issue, not just the symptoms.
4) Determine Corrective Actions
Once the root cause is identified, a corrective action plan is developed to fix the problem and ensure it doesn’t happen again.
5) Determine Preventive Actions (if applicable)
If the issue identified can lead to other problems, preventive actions are taken to eliminate the cause of potential future issues.
6) Implement the Actions
Corrective and preventive actions are implemented as per the plan. This could include process changes, equipment upgrades, training programs, etc.
7) Effectiveness Verification
After implementation, the effectiveness of the corrective and preventive actions is verified through further testing, monitoring, or audits.
Closure:
Once it’s confirmed that the actions have been effective, the CAPA process is closed, and all documentation is finalized.
Key Elements of an Effective CAPA System
1) Documentation
Every CAPA activity must be well-documented to provide evidence of compliance. A report typically includes:
Problem description
Root cause analysis
Corrective and preventive action plans
Implementation records
Effectiveness verification
Approval and closure of the CAPA
2) Root Cause Analysis
Identifying and addressing the root cause is critical to ensure that corrective actions prevent the recurrence of the issue.
3) Cross-Functional Teams
CAPA often involves a team of subject matter experts from different departments (e.g., Quality Assurance, Production, Engineering) to ensure a thorough investigation and effective solution.
4) Management Oversight
Senior management must oversee the CAPA process to ensure timely action and resource allocation.
5) Regulatory Compliance
CAPA is required by regulatory agencies to maintain product quality and safety. It helps companies demonstrate that they have effective systems in place for managing deviations and improving processes.
Tools Used in Corrective Action and Preventive Action
Several tools and methodologies can be used for root cause analysis and problem-solving within CAPA, including:
1) 5 Whys Analysis: Repeatedly asking “Why?” to drill down to the root cause of a problem.
2) Fishbone Diagram (Ishikawa): A visual tool that categorizes potential causes of a problem.
3) FMEA (Failure Mode and Effects Analysis): A systematic method for identifying and prioritizing risks in processes.
4) Pareto Analysis: A statistical technique that helps prioritize issues by identifying the most frequent or significant problems.
Examples of CAPA in Pharmaceutical Industry
1) Corrective Action Example
A batch of tablets fails dissolution testing. Investigation reveals that the mixing time during production was not adequate. The corrective action involves updating the standard operating procedure (SOP) to increase the mixing time, retraining staff, and implementing stricter monitoring of the process to ensure compliance.
2) Preventive Action Example
During an internal audit, it is discovered that several pieces of equipment have missed scheduled maintenance checks. A preventive action plan is put in place to improve the maintenance scheduling system and include automatic reminders, preventing equipment failures in the future.
Conclusion
CAPA is a vital tool for ensuring quality, compliance, and continuous improvement in the pharmaceutical and other regulated industries. It helps companies systematically investigate and address both actual and potential problems, ultimately enhancing product quality and patient safety. Proper implementation of CAPA is critical to meeting regulatory standards and maintaining a robust quality management system.
Frequently Asked Questions (FAQs)
What is CAPA in the pharmaceutical industry, and why is it important?
CAPA (Corrective Action and Preventive Action) is a systematic process used in the pharmaceutical industry to identify, correct, and prevent issues that affect product quality, safety, and compliance. It is essential because it helps companies address deviations, defects, and non-conformances, ensuring that root causes are eliminated to prevent recurrence. It ensures regulatory compliance with authorities like the FDA and WHO, maintains product quality, and protects patient safety.
What are the key steps involved in the CAPA process?
The CAPA process typically involves the following steps:
Problem Identification – Recognizing and documenting the issue.
Root Cause Analysis – Identifying the underlying cause of the problem.
Corrective Action – Developing and implementing actions to eliminate the root cause.
Preventive Action – Taking steps to prevent similar issues in the future.
Verification of Effectiveness – Monitoring the process to ensure the actions have successfully addressed the problem and prevent recurrence.
Documentation and Closure – Keeping records of the CAPA process for regulatory compliance and closing the CAPA when verified.
How is the effectiveness of CAPA verified in the pharmaceutical industry?
CAPA effectiveness is verified by monitoring the affected process or system after implementing corrective and preventive actions. This involves:
1) Auditing or inspecting the updated process to confirm it functions correctly.
2) Testing to ensure the issue has been resolved (e.g., product quality testing).
3) Reviewing records to ensure no recurrence of the issue.
4) Monitoring KPIs to assess long-term effectiveness. Verification must be documented in a CAPA report and approved before the officially closed.

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



