Learn about Propylene Glycol vs Ethylene Glycol means the key differences between propylene glycol and ethylene glycol, including their chemical properties, uses, toxicity levels, and safety. Discover which is safer for various applications and industries.
Table of Contents
Introduction
Propylene glycol (PG) and ethylene glycol (EG) are two common chemicals widely used in various industrial and consumer applications, especially as antifreeze and heat transfer fluids. While these glycols share similar properties, such as being colorless, odorless liquids, they differ significantly in their chemical structures, toxicity, and usage.
Understanding these differences is important for selecting the right compound for specific applications, particularly where safety and environmental impact are concerns.
Chemical Structure
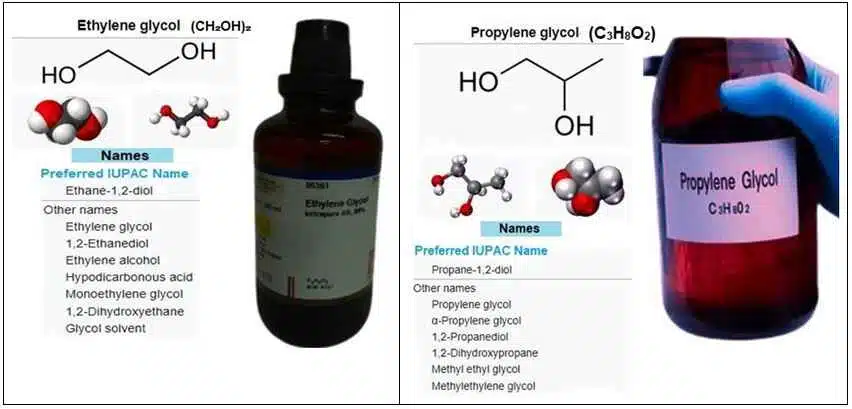
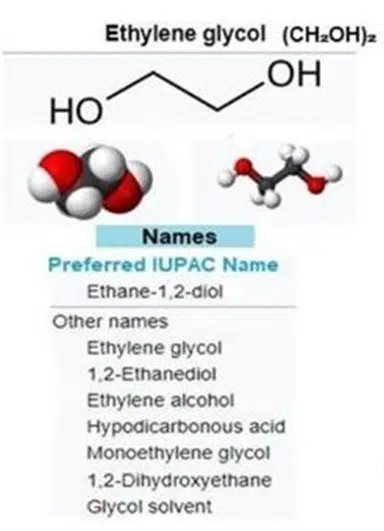
Ethylene Glycol (EG):
EG is a small molecule with the chemical formula C₂H₆O₂. Structurally, it has two hydroxyl groups (-OH), making it a diol.
This simple dihydric alcohol is known for its high solubility in water and ability to depress the freezing point of water, which is why it’s commonly used as an antifreeze.
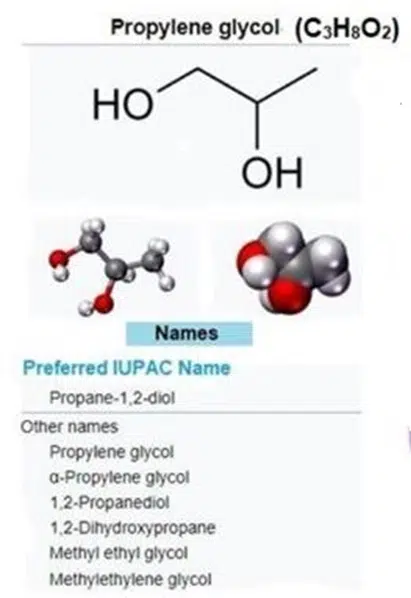
Propylene Glycol (PG):
PG has the chemical formula C₃H₈O₂. It contains three carbon atoms and two hydroxyl groups, like ethylene glycol, but differs in its carbon chain structure.
PG is classified as a generally recognized as safe (GRAS) substance by the FDA and is widely used in food, pharmaceuticals, and cosmetics.
Toxicity and Safety
Ethylene Glycol (EG):
One of the biggest concerns with ethylene glycol is its toxicity.
It is highly poisonous if ingested, even in small quantities, and can cause severe harm to the central nervous system, kidneys, and heart.
Ethylene glycol poisoning often results in metabolic acidosis, kidney failure, and even death if not treated quickly.
Due to these risks, ethylene glycol is usually used in applications where human exposure is minimal, such as automotive antifreeze or industrial cooling systems.
Propylene Glycol (PG):
Propylene glycol is considered much safer than ethylene glycol and is approved for use in various consumer products.
It has low toxicity, and in fact, it is used as a food additive (E1520) and in pharmaceuticals as a solvent for oral, injectable, and topical medications.
Although it can cause minor irritation or allergic reactions in high concentrations, it is generally safe for human exposure, even in everyday items such as cosmetics, lotions, and processed foods.
Applications
Ethylene Glycol Uses:
Antifreeze:
Ethylene glycol is the primary ingredient in automotive antifreeze and coolants. Its ability to lower the freezing point of water makes it ideal for keeping engines from freezing in cold temperatures while also preventing overheating in warm climates.
Heat Transfer Fluid:
EG is also used in heating and cooling systems, such as HVAC systems and industrial refrigeration units. It offers superior thermal conductivity and is effective in extreme temperature conditions.
Plastics Manufacturing:
EG is a key raw material in the production of polyethylene terephthalate (PET), a plastic commonly used in making bottles, textiles, and packaging materials.
Propylene Glycol Uses:
Food and Pharmaceuticals:
PG is commonly used in food products as a humectant, preservative, and flavor carrier. In the pharmaceutical industry, it serves as a solvent for active ingredients in injectable, oral, and topical medications.
Cosmetics and Personal Care Products:
PG is found in many personal care products like moisturizers, shampoos, deodorants, and toothpastes due to its ability to retain moisture, improve product stability, and act as a skin conditioning agent.
Antifreeze (Non-Toxic):
Although less efficient than EG in heat transfer properties, PG is used in antifreeze products for applications where toxicity is a concern, such as in food processing, HVAC systems in hospitals, and systems requiring environmentally friendly solutions.
Performance Comparison:
Freezing Point Depression: Both ethylene glycol and propylene glycol are effective at lowering the freezing point of water, but ethylene glycol generally performs better in extreme cold temperatures. As a result, EG-based antifreeze is more effective in colder climates, while PG-based antifreeze is preferred in areas where environmental or human safety is a priority.
Thermal Conductivity: Ethylene glycol has better thermal conductivity than propylene glycol, making it a superior choice for industrial applications that require efficient heat transfer, such as in automotive cooling systems.
Viscosity: Propylene glycol tends to have higher viscosity than ethylene glycol, which can affect flow rates in certain systems. This makes EG a more suitable option where lower viscosity and higher flow efficiency are required.
Environmental Impact
Ethylene Glycol:
Due to its toxicity, ethylene glycol can be harmful to wildlife if spilled into the environment, and it must be disposed of properly to prevent contamination. It breaks down in the environment but can have serious short-term effects on aquatic life if not handled responsibly.
Propylene Glycol:
Propylene glycol is biodegradable and has a much lower environmental impact than ethylene glycol. Because it is non-toxic to humans and animals, it is often the preferred choice for environmentally sensitive applications, such as in food processing plants or areas near water sources.
Conclusion
When choosing between propylene glycol and ethylene glycol, the decision hinges on the balance between performance requirements and safety concerns. Ethylene glycol offers superior thermal properties and is more effective in cold climates, making it a go-to for industrial applications like automotive antifreeze. However, its high toxicity makes it less suitable for environments where human or animal exposure is possible.
Propylene glycol, on the other hand, is a safer alternative, often used in food, cosmetics, and pharmaceutical applications. Its lower toxicity and biodegradability make it a more environmentally friendly option, although it sacrifices some efficiency in thermal transfer and viscosity.
In summary, while both glycols serve similar purposes, their differences in toxicity, performance, and environmental impact dictate their appropriate use in various industries.
Frequently Asked Questions (FAQs)
What are propylene glycol and ethylene glycol used for?
Propylene glycol is commonly used in food, cosmetics, pharmaceuticals, and as a less-toxic antifreeze. It is also used in vape liquids, as a moisturizer in lotions, and in air sanitizers.
Ethylene glycol is primarily used as an industrial coolant, antifreeze in vehicles, and in deicing fluids. Due to its toxicity, it is not used in food or cosmetic products.
How are propylene glycol and ethylene glycol chemically different?
Both are alcohols and belong to the glycol family, but their chemical structures are slightly different. Propylene glycol is a three-carbon alcohol (C₃H₈O₂), whereas ethylene glycol is a two-carbon alcohol (C₂H₆O₂). This difference affects their toxicity and physical properties.
Which one is more toxic, propylene glycol or ethylene glycol?
Ethylene glycol is far more toxic than propylene glycol. Ingesting ethylene glycol can lead to severe poisoning, kidney failure, and even death. On the other hand, propylene glycol is generally recognized as safe (GRAS) by the FDA for use in food and cosmetics and has very low toxicity.
Can propylene glycol and ethylene glycol be used interchangeably as antifreeze?
No, ethylene glycol is a more efficient antifreeze because it has a lower freezing point and better heat transfer properties. However, propylene glycol is often used as a safer alternative in applications where toxicity is a concern, such as in food processing systems, breweries, and HVAC systems in residential areas.
How do propylene glycol and ethylene glycol impact the environment?
Both glycols can have negative environmental impacts if released in large quantities, but ethylene glycol is more hazardous due to its toxicity. Propylene glycol, being less toxic, breaks down more easily and poses less risk to aquatic life and the environment when used responsibly.
Related Topic:
Propylene Glycol in Pharmaceuticals
Difference Between Drugs and Medicines
Difference Between Water and Moisture Content

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



