In the pharmaceutical sector, validation is an essential part of quality assurance. It entails a methodical process of creating written proof that a certain method, tool, system, or equipment reliably yields outcomes that adhere to predefined standards and quality characteristics.
Table of Contents
Importance of Validation
Regulatory Compliance: Compliance with regulatory agencies such as the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and other global authorities is mandatory. Validation ensures adherence to Good Manufacturing Practices (GMP) and other regulatory guidelines.
Product Quality and Safety: Validation assures that products are of the highest quality and safe for consumer use by minimizing variability and errors in production processes.
Risk Management: Identifies potential risks and implements controls to mitigate them, ensuring consistent product performance.
Cost Efficiency: Reduces waste, rework, and recalls by ensuring processes are efficient and effective from the outset.
Types of Validation
1. Process Validation:
Demonstrates that a process consistently yields products meeting predetermined quality criteria.
i) Prospective Validation: Conducted before the new product is released for sale, based on a predetermined plan.
ii) Concurrent Validation: Performed during actual production when previous validation data is insufficient.
iii) Retrospective Validation: Based on historical data of existing products and processes.
iv) Revalidation: Ongoing validation to ensure processes remain in control over time.
2. Analytical Method Validation:
Ensures that analytical testing methods are accurate, precise, specific, and robust.
3. Cleaning Validation:
Confirms that cleaning procedures effectively remove residues to acceptable levels, preventing cross-contamination.
4. Computer System Validation (CSV):
Validates that computer systems perform reliably and consistently as intended.
5. Equipment and Facility Validation:
i) Installation Qualification (IQ): Verifies that equipment and systems are installed according to specifications.
ii) Operational Qualification (OQ): Tests that equipment operates within specified parameters.
iii) Performance Qualification (PQ): Ensures that equipment consistently performs according to operational specifications under real conditions.
Regulatory Guidelines
1. FDA Guidance:
21 CFR Part 211: Outlines GMP for finished pharmaceuticals.
Process Validation: General Principles and Practices (2011): Emphasizes a lifecycle approach to process validation.
2. ICH Guidelines:
ICH Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients.
ICH Q8 (R2): Pharmaceutical Development.
ICH Q9: Quality Risk Management.
ICH Q10: Pharmaceutical Quality System.
3. EU Guidelines:
EudraLex Volume 4: Guidelines for GMP for medicinal products for human and veterinary use.
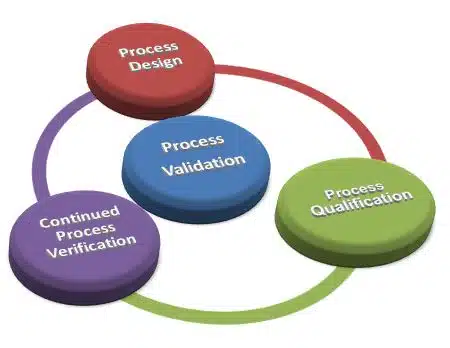
Lifecycle Approach
Modern validation strategies adopt a lifecycle approach consisting of:
1. Process Design: Developing and understanding the manufacturing process during product development.
2. Process Qualification: Confirming that the process design is capable of reproducible commercial manufacturing.
3. Continued Process Verification: Ongoing assurance that the process remains in a state of control during routine production.
Key Documents
1. Validation Master Plan (VMP): A comprehensive document outlining the validation strategy, including scope, responsibilities, and schedule.
2. Standard Operating Procedures (SOPs): Detailed instructions to ensure consistency in performing tasks.
3. Validation Protocols: Detailed plans describing the validation process, including objectives, responsibilities, procedures, and acceptance criteria.
4. Validation Reports: Documents summarizing the validation activities, results, deviations, and conclusions.
5. Change Control Management: Procedures to manage changes in processes, equipment, or systems that could impact validated status.
6. Training and Competency: Ensuring that personnel involved in validation activities are adequately trained and competent.
Current Trends and Best Practices
1. Risk-Based Approach: Prioritizing validation efforts based on the potential impact on product quality and patient safety.
2. Quality by Design (QbD): Integrating quality into product design and development, emphasizing understanding processes and controlling variability.
3. Data Integrity: Ensuring accuracy, consistency, and reliability of data throughout its lifecycle, in compliance with ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available).
4. Automation and Digitalization: Utilizing advanced technologies for process control, data management, and improving efficiency.
5. Continuous Manufacturing: Transitioning from traditional batch processes to continuous production, requiring novel validation approaches.
Challenges in Validation program
1. Complexity of Biologics: Biopharmaceuticals present unique validation challenges due to their complex nature and variability.
2. Regulatory Changes: Keeping up with evolving regulatory requirements across different regions.
3. Global Supply Chains: Ensuring consistent validation practices across multinational operations.
4. Technological Advances: Adapting validation practices to accommodate new technologies like single-use systems and personalized medicine.
Conclusion
A robust validation program is essential for ensuring that pharmaceutical products are consistently produced to the highest quality standards. It requires meticulous planning, execution, and documentation, aligned with regulatory expectations and industry best practices. By adopting a lifecycle approach and focusing on continuous improvement, pharmaceutical companies can enhance product quality, ensure patient safety, and maintain regulatory compliance.
References
DA Guidance for Industry: Process Validation: General Principles and Practices (2011)
ICH Guidelines Q7, Q8(R2), Q9, and Q10
EudraLex Volume 4: EU Guidelines for Good Manufacturing Practice

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



