Root Cause Analysis (RCA) is a systematic approach used in the pharmaceutical industry to identify the underlying causes of problems or deviations that impact product quality, safety, or regulatory compliance. By uncovering the true origins of issues, Root Cause Analysis enables organizations to implement effective corrective and preventive actions (CAPA), ensuring the reliability of processes and maintaining compliance with stringent industry regulations.
Importance of Root Cause Analysis (RCA) in Pharmaceuticals
In the pharmaceutical industry, deviations, non-conformances, and out-of-specification (OOS) results can compromise product quality and patient safety.
RCA plays an important role in:
Ensuring Product Quality: Identifying and addressing root causes helps maintain the consistent quality of pharmaceutical products.
Regulatory Compliance: Authorities like the FDA and EMA require thorough investigation of quality issues, making Root Cause Analysis a critical compliance tool.
Minimizing Risks: By resolving underlying issues, RCA reduces the likelihood of recurring problems, enhancing operational efficiency and safety.
Cost Reduction: Preventing repeat issues saves resources, time, and money associated with recalls, rework, or regulatory penalties.
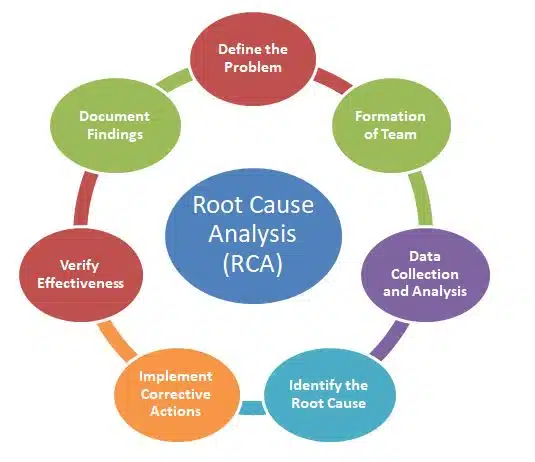
Steps for Root Cause Analysis Technique
The RCA process involves a structured series of steps to ensure thorough investigation and resolution of issues:
1. Define the Problem
Clearly document the issue, including its nature, location, time of occurrence, and potential impact.
Example: An OOS result in the dissolution test of a drug batch.
2. Formation of Team
Form a multidisciplinary team with expertise in the relevant areas (e.g., quality control, manufacturing, and engineering).
Ensure effective communication and collaboration within the team.
3. Data Collection and Analysis
Gather all relevant data, including batch records, equipment logs, and environmental monitoring reports.
Use tools like process mapping or fishbone diagrams to visualize potential causes.
4. Identify the Root Cause
Use structured methodologies like the “5 Whys” or Failure Mode and Effects Analysis (FMEA) to drill down to the fundamental cause.
Example: If an equipment failure is identified, ask why it occurred and explore underlying maintenance or design issues.
5. Implement Corrective Actions
Create a CAPA plan to deal with the root cause of the issue.
Ensure the actions are specific, measurable, achievable, relevant, and time-bound (SMART).
6. Verify Effectiveness
Monitor the implementation of corrective actions and evaluate their effectiveness in preventing recurrence.
Perform periodic audits to ensure sustained improvement.
7. Document Findings
Maintain detailed records of the RCA process, including problem descriptions, investigation methods, and implemented actions.
Documentation ensures transparency and serves as evidence for regulatory audits.
Tools Commonly Used for Root Cause Analysis
Fishbone Diagram (Ishikawa):
Categorizes potential causes into areas such as personnel, equipment, materials, methods, environment, and measurement.
Steps:
Define the problem and write it at the “head” of the diagram.
Draw a “spine” and categorize possible causes (e.g., People, Methods, Machines, Materials, Environment).
Brainstorm specific causes within each category.
Analyze and prioritize the most likely causes.
Application: Commonly used in quality control and process improvement.
5 Whys Analysis:
The 5 Why method is a problem-solving technique used in Root Cause Analysis to identify the underlying cause of a problem. By repeatedly asking “Why?”—generally five times— You can follow the causal chains that lead to the root issue.
Steps:
Clearly define the problem.
Ask “Why did this happen?” and record the answer.
Repeat the question for each subsequent answer (typically 5 times or until the root cause is found).
Implement solutions addressing the root cause.
Application: Frequently used in Six Sigma and Lean manufacturing procedures.
Pareto Analysis:
Helps prioritize causes by identifying the most significant factors. Based on the 80/20 rule, Pareto Analysis focuses on identifying the few vital causes that contribute to the majority of a problem.
Steps:
List and quantify problems or causes.
Sort them by effect or frequency in descending order.
Create a Pareto chart (bar graph with cumulative percentage).
Prioritize solutions based on the “vital few” causes.
Application: Helps prioritize efforts in quality control and process improvement.
Failure Mode and Effects Analysis (FMEA):
FMEA is a systematic, proactive method to identify potential failure modes, assess their impact, and prioritize actions to reduce risks.
Steps:
Identify the system, process, or product components.
List potential failure modes for each component.
Analyze the effects of each failure mode.
Determine severity, occurrence, and detection ratings to calculate the Risk Priority Number (RPN).
Address high-priority risks with mitigation plans.
Application: Used in manufacturing, design, and service industries.
Fault Tree Analysis (FTA):
To determine the probable reasons behind a system failure, FTA is a top-down, deductive analytical technique. It visually represents the logical relationships between various subsystems and components that can lead to the undesired event (the “top event”).
Steps:
Define the top event (failure or undesired event).
Break it down into contributing causes using logic gates (AND, OR).
Identify the root causes at the lowest levels.
Mitigate or eliminate the identified risks.
Application: Widely used in safety and reliability engineering.
Challenges in Root Cause Analysis Implementation
Incomplete Data: Lack of sufficient data can hinder accurate identification of root causes.
Time Constraints: Tight production schedules may limit thorough investigations.
Human Bias: Preconceived notions can skew the analysis, leading to incorrect conclusions.
Insufficient Training: Ineffective use of RCA tools due to inadequate training can compromise outcomes.
Best Practices for Effective Root Cause Analysis
Foster a Quality Culture: Encourage a culture of openness and continuous improvement.
Invest in Training: Provide training on RCA tools and methodologies for employees.
Engage Cross-Functional Teams: Leverage diverse expertise for comprehensive analysis.
Focus on Prevention: Prioritize preventive measures over reactive solutions.
Leverage Technology: Use digital tools for data analysis and documentation to streamline the Root Cause Analysis process.
Conclusion
Root Cause Analysis is a vital component of quality management in the pharmaceutical industry. By systematically identifying and addressing the underlying causes of issues, RCA helps ensure product quality, safeguard patient safety, and maintain regulatory compliance. Implementing robust Root Cause Analysis processes, supported by skilled teams and effective tools, enables organizations to enhance operational efficiency and build trust with stakeholders. In an industry where the stakes are high, RCA is not just a problem-solving method but a commitment to excellence.
Frequently Asked Questions (FAQs)
What is Root Cause Analysis (RCA) and why is it important?
Root Cause Analysis is a systematic process used to identify the underlying causes of a problem or failure rather than merely addressing its symptoms. By understanding and addressing the root cause, organizations can prevent recurrence, improve processes, and enhance overall efficiency and reliability, making RCA a critical tool in quality management and continuous improvement.
What are the key steps involved in conducting an Root Cause Analysis?
The Root Cause Analysis process typically involves identifying and clearly defining the problem, collecting relevant data, analyzing the information to pinpoint the root causes, developing corrective actions, and implementing and monitoring these actions to ensure the issue does not recur. Tools such as the “5 Whys,” Fishbone Diagram, or Fault Tree Analysis are often used to aid in this structured investigation.
What are the challenges in conducting an effective Root Cause Analysis?
Challenges in Root Cause Analysis include inadequate data collection, biased perspectives, lack of collaboration among team members, and insufficient follow-through on corrective actions. Overcoming these challenges requires a thorough investigation, open communication, cross-functional team involvement, and a strong commitment to implementing sustainable solutions.

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



