Learn how to write a SOP for Internal Audit and self inspection in the pharmaceutical industry.
Table of Contents
1. PURPOSE
To describe a standard procedure to give a planned and systematic examination and check of a system, procedure or operation in order to monitor complies with and the effectiveness of established standards and to allow for improvement and corrective measure where required.
2. SCOPE
This SOP is applicable for Internal Audit procedure of all departments in Pharmaceuticals.
3. RELATED DOCUMENTS
Nil
4. PRECAUTIONS
Put on the proper clothes and safety equipment for every location that is being audited.
5. RESPONSIBILITIES
5.1 The Quality Assurance executive/manager is responsible for preparing the audit schedule.
5.2 The head of the Quality Assurance consist an Audit team, issuance of annual audit program, implementation of each audit schedule, and follow up of audit observations.
5.3 The members of the audit team are in charge of conducting the audit, setting up the meeting for discussion, and submitting the audit report.
5.4 Responsible person for the action is responsible to the carry out the suggested corrective action within agreed time & returns the audit observation report to Quality Assurance Department.
6. ACCOUNTABILITY
Head of the Quality Assurance Department
7. PROCEDURE
7.1 Issue an annual audit program covering all sections / departments which will be audited.
7.2 Constitute an Internal Quality Audit Team of at least 3 (three) members. The team will include personnel not normally employed in that area.
7.3 Brief the personnel employed in an audit team about auditing procedures and prepare personnel prior to audit by a review of cGMP, other relevant guidelines and previous inspection report.
7.4 Inform the specific date of the audit to the head of department to be audited so that the department /section is sufficiently prepared beforehand.
7.5 The Audit Team will audit respective area According to ‘Audit Check List’ (Annexure-1)
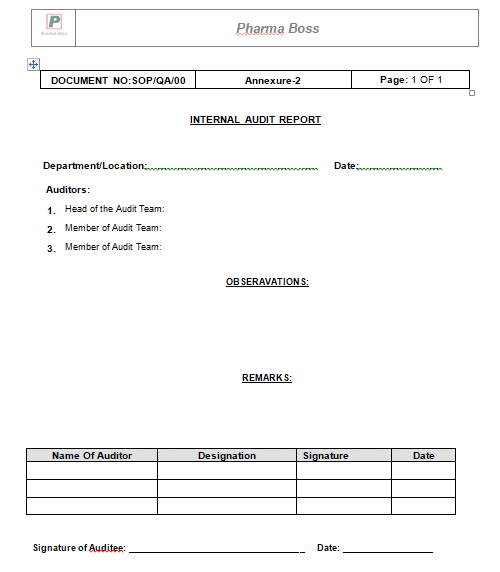
7.6 Carry out the audit & note down the observations on the ‘Internal Audit Report’ (Annexure-2).
7.7 Take signature of the Auditor & Auditee on the internal Audit report.
7.8 Arrange an audit observations discussion meeting within a week & invite the General Manager, Quality Operations, concerned departmental head of Quality Assurance.
7.9 Discuss the corrective & preventive action of the each observation & note down the actions by defining the persons responsible for action with time limit.
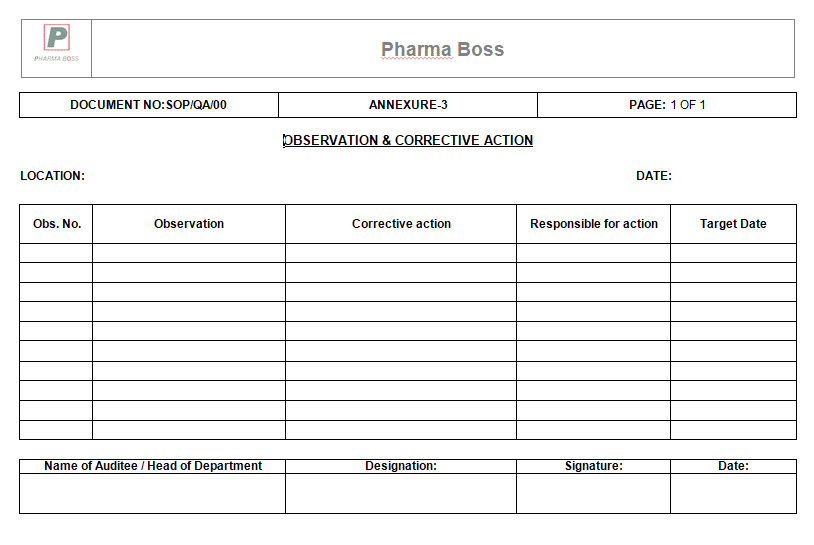
7.10 Prepare the final audit report (Annexure-3) within 7 (Seven) working days from the discussion meeting.
7.11 Take signature of the Auditee’s departmental head on the final Audit report.
7.12 Send the final report to Quality Assurance Department & Share copy of the report with each auditor and auditee’s departmental head.
7.13 Follow up the implementation of the action systematically to ensure this audit observation report.
7.14 Carry out audit for all areas at least once in a year
Read Also: SOP for Concurrent Process Validation
8. ABBREVIATIONS
8.1 SOP: Standard Operating Procedure
8.2 cGMP: Current Good Manufacturing Practice
9. ANNEXURES
9.1 Annexure-1: Audit Check List
9.2 Annexure-2: Internal Audit Report
9.3 Annexure-3: Observations & Corrective action form
REFERENCE: WHO

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.




Thanks