Learn how to write an SOP for the Planned Preventive Maintenance schedule of process and utility equipment in the pharmaceutical plant.
Table of Contents
1.0 PURPOSE
The purpose of ‘Preventive Maintenance’ is to carry out maintenance on equipment in accordance with the manufacturer’s requirements or recommendations to maintain the performance of the system.
2.0 SCOPE
This SOP is applicable to all process and utility equipment recommended for preventive maintenance used in pharmaceuticals.
3.0 ASSOCIATED DOCUMENTS
3.1 SOP for Guidelines for Maintenance Procedure.
3.2 SOP for Permit to Work—General.

4.0 DEFINITIONS
Planned Preventive Maintenance (PPM): This deals with the servicing of equipment that has no observed faults to minimize the risk of future breakdowns.
5.0 RESPONSIBILITIES
5.1 The officer or executive in engineering is responsible for implementing the preventive maintenance schedule.
5.2 The head of Engineering is responsible for ensuring that the preventive System
implemented.
Read Also: SOP of SOP in Pharmaceutical
6.0 PRECAUTIONS
6.1 All safety procedures are applicable.
6.2 The maintenance must be carried out by trained engineering staff or an appropriate contractor.
7.0 PROCEDURE
7.1 Schedule Preparation
7.1.1 Identify and assign all equipment ID, Tag No., Description of equipment, related SOP, and EHF no. and fill them in the Equipment table of both Process and Utility Software.
7.1.2 Identify all the different types of maintenance frequencies from the related equipment’s Preventive Maintenance SOPs and assign them Frequency ID (FI) nos. (i.e. 1, 2, 3 …)
7.1.3 Place all the maintenance actions of equipment on the Main Table spreadsheet from related Preventive Maintenance SOPs.
7.2 Schedule Generation
7.2.1 Take printouts of all the ‘Weekly Listing’ data every Saturday and distribute them to respective Engineering Staff.
7.2.2 Take printouts of all the ‘Task Sheets’ every Saturday and distribute them to respective Engineering Staff for implementation
7.3 Schedule Execution
7.3.1 Check that all the actions mentioned in the ‘Task Sheet’ are done properly and put a tick (√) Mark on the related box. If not, put a comment below.
7.3.2 Ensure that the duration between the due date & work execution date does not exceed (from the due date):
| Period | Days |
| Weekly | 7 |
| Monthly | 30 |
| Three Monthly | 90 |
| Six Monthly | 180 |
7.3.3 If the execution date exceeds the allowable limit, put proper justification for this in the ‘Comment’ section.
7.4 Record Data
7.4.1 Collect the entire ‘Task sheet after completion. Check that all the sheets are Properly Signed and dated.
7.4.2 Put the completion date of the ‘Task Sheets’ on the PPM ‘Qdata-02’ Queries table against ‘Task Sheet’ Record ID no for the respective type of PPM (Process & Utility).
Write the ‘Task Sheet’ comments (if any) following the sequential operation of the Main Menu, Data Entry, Equipment, Records, Record ID, and Comments window. This will work as an electronic backup of Maintenance History.
7.4.3 Place the completed ‘Task Sheets’ in the respective Engineering History Files ‘Servicing History’ section in chronological order.
8.0 ABBREVIATIONS
8.1 SOP: Standard Operating Procedure
8.2 PPM: Planned Preventive Maintenance
9.0 ANNEXURES
9.1 Annexure 1: Task Sheet
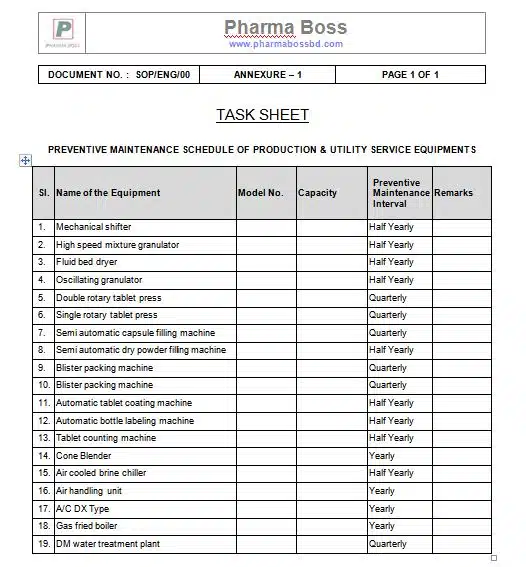
Frequently Asked Questions (FAQs)
What is Planned Preventive Maintenance?
Planned Preventive Maintenance in the pharmaceutical industry refers to scheduled upkeep of equipment and systems to prevent failures. It ensures optimal functioning, reduces downtime, and maintains quality standards, critical for safe and efficient drug production.

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



