Learn how to write a SOP for sampling method of raw materials in sampling booth.
1. PURPOSE
The purpose of this procedure is to describe sampling method of raw materials for ensuring the identity and quality of raw materials by subsequent testing.
2. SCOPE
This procedure is applicable for sampling method of raw materials in warehouse of this pharmaceutical.
3. ASSOCIATED DOCUMENTS
SOP for operating procedure of Sampling Booth.
4. PRECAUTIONS
4.1 Use gloves, mask, hair and eye protector during sampling.
4.2 Start sampling booth 30 minutes before sampling starts.
4.3 Always collect raw materials samples in the sampling booth.
4.4 Wear clean dress before sampling. Charge used clothes after each & every active material sampling.
4.5 Avoid the contact of chemicals with skin and clothing.
4.6 Avoid the inhalation of vapor and dust.
4.7 Take care during sampling of toxic and flammable liquid.
4.8 Always sample raw material taking precaution according of Material safety data sheet. Always use clean and dry utensils for sampling.
4.9 For microbiological testing use sterilized utensil for collecting sample.
4.10 Ensure that sampling booth is clean and free from materials concerned to previous sampling.
5. RESPONSIBILITIES
5.1 Executive, Quality Control or sampler is responsible for carrying out the sampling procedure.
5.2 Cleaner is responsible for cleaning.
6. ACCOUNTABILITY
Head of the Quality Assurance Department
7. PROCEDURE
7.1 Receive” Goods Received Note” (GRN)
7.2 Entry your company batch No., name of raw material, lab control no. etc. for the each batch of the supplier batch no. in a register.
7.3 Select number of containers/bags as per sampling plan and inform warehouse to send the raw material containers near the sampling booth.
7.4 Label the sampling container properly.
7.5 Start-up the sampling booth following the associated SOP.
7.6 Check the physical condition of containers/bags.
7.7 Open the container and check the physical condition of the raw material such as uniformity of color, appearance or any lump formation etc.
7.8 Collect top, middle and bottom of the container about 50 gm. from for solid sample and 500ml for liquid sample. Repair the sampling point properly and close the container.
7.9 Check the label of the sample container and verify with respective container.
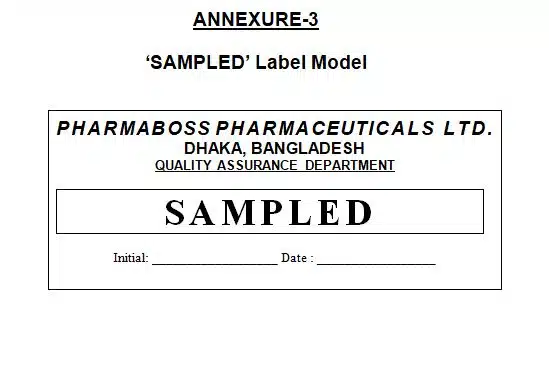
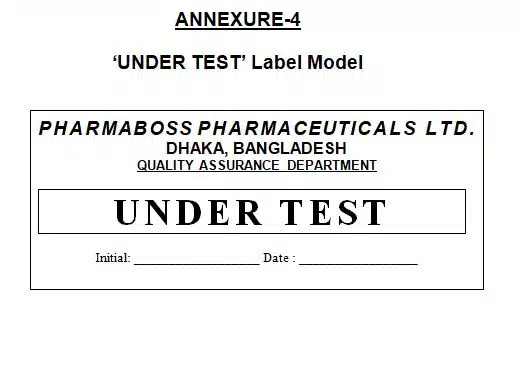
7.10 Prepare and affix the ‘Sampled’ label only sampled container and ‘Under Test’ label each and every container of the batch.
7.11 Switch off the sampling booth and affix ‘To be cleaned’ label.
7.12 Inform warehouse to take the raw material from sampling booth to quarantine area.
7.13 Hand over the samples to Executive, QC along with necessary documents.
7.14 Keep retention sample from each lot of raw material with proper labeling at least twice the quantity required to carry out all tests.
7.15 Sampling Plan
7.15.1 Determine the number of containers of Excipients for sampling by √n + 1 for each batch. Where N = total no. of container.
7.15.2 Sample each container of active raw material for identity test.
7.15.3 Take sufficient samples to permit repeat test (if necessary) and retention sample.
Sampling note:
For Solid
a) If the materials appear uniform, take the sample by turning the upper few inches over with a scoop
b) If the material is in paper bag, take the sample by cutting a ‘V’ on the bag, push the spear and take sample into a labeled container. Repair the on the bag properly with self-adhesive tape.
For Liquid
Gently stir the liquid with a suitable dipstick, take samples from the middle of the container and transfer to a labeled container.
For Damaged Container
Sample from each damaged container separately.
Read Also: SOP for Cleaning of Sampling Tools
8. ABBREVIATIONS
8.1 SOP: Standard Operating Procedure
8.2 GRN: Goods Received Note
8.3 QC: Quality Control
9. ANNEXURES
9.1 Annexure-1: Raw Material Sampling Log book
9.2 Annexure-2: Raw Material Register Log book
9.3 Annexure-3: ‘SAMPLED’ Label Model
9.4 Annexure-4: ‘UNDER TEST’ Label Model
SOP For Sampling Method of Raw Materials, Annexure-1: Raw Material Sampling Log book

SOP For Sampling Method of Raw Materials, Annexure-2: Raw Material Register Log book
ANNEXURE-2 Raw Material Register Log book
| Date | Name of the Raw materials | Batch No. | GRN No. | Lab Control No | Name of the Manufacturer | Supplier Batch No. | Mfg. date | Exp. Date | Sampled by | Tested by | Quantity | Remarks |
SOP For Sampling Method of Raw Materials, Annexure-3: ‘SAMPLED’ Label Model
SOP For Sampling Method of Raw Materials, Annexure-4: ‘UNDER TEST’ Label Model

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



