Learn how to write a (Standard Operating procedure) SOP for training records of employees in the pharmaceuticals.
1. PURPOSE
To describe a standard procedure of all training conducted in the all employee of this Pharmaceuticals is recorded in proper schedule.
2. SCOPE
This SOP is applicable for all employees working at Pharma Boss Pharmaceuticals Limited.
3. RELATED DOCUMENTS
All SOP and documents associated with training.
4. PRECAUTIONS
Nil
5. RESPONSIBILITIES
5.1 A nominated person of each department is responsible for maintaining training records files.
5.2 Departmental Heads are responsible to identify the requirement of training, of their employees and he is also responsible to arrange training programmes.
Read Also : SOP for Concurrent Process Validation
6. ACCOUNTABILITY
Head of the Quality Assurance Department: Responsible for implementation, arrange and evaluation of training programmes.
7. PROCEDURE
7.1 Open a file for each new employee.
7.2 Arrange the file into the following sections-
7.2.1 Training Summary
7.2.2 General GMP Training and cGMP Training.
7.2.3 Departmental SOP Training (SOP based)
7.2.4 Internal Vocational Training
7.2.5 External Vocational Training
7.2.6 Individual Profile (CV, job description etc.)
7.2.7 Copies of any Professional/ Technical Qualifications.
7.3 Document 7.2.2 to 7.2.5 on the Training Record along with any relevant supporting documents attached (e.g. completed questionnaires, certificates for external training etc.)
7.4 Consider the person’s job description and identify specific training requirements to enable that person to perform the job competently.
7.5 Implement training in a logical and sequential manner.
7.6 Document each and every training on the Training record form.
7.7 Ensure that it is completed fully, particularly where an external Commissioning Engineer/trainer provides the training.
7.8 Keep the completed form in the individual’s training records file.
7.9 Update the Training Summary sheet with details of the training, and keep it on the top of the file.
7.10 Review each item of internal training on an annual basis. The review can simply be an extension for a further year if there is evidence that the trainee has performed the duties competently in the current period. If it is considered that the trainee requires fresher training or if the procedure has been changed, then they must be retrained and the training documented. In the case of revisions to procedures, training must be conducted immediately after the new procedure is issued.
7.11 Note that all training records may be subject to inspection by both internal and external
8. ABBREVIATIONS
8.1 SOP: Standard Operating Procedure
8.2 GMP: Good Manufacturing Practice
8.3 cGMP: Current Good Manufacturing Practice
9. ANNEXURES
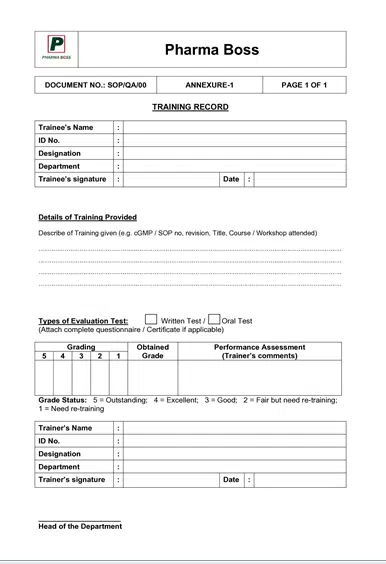
9.1 Annexure-1: Training Record

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



