A Validation Schedule is a documented plan that outlines all the validation activities a pharmaceutical manufacturing facility must perform over a specific period. It ensures that all critical processes, equipment, systems, and procedures are consistently operating according to predefined specifications. A robust validation schedule is essential for maintaining compliance with Good Manufacturing Practices (GMP) and ensuring product quality and safety.
Table of Contents
Here are the details of a typical validation schedule:
1. Purpose of the Validation Schedule
The validation schedule ensures that all necessary validation activities, including installation qualification (IQ), operational qualification (OQ), performance qualification (PQ), and process validation, are planned and executed within a defined timeframe.
2. Scope of the Validation Schedule
The validation schedule covers:
a) Equipment and machinery used in production.
b) Manufacturing processes (e.g., mixing, granulation, tableting).
c) Analytical methods used in quality control.
d) Cleaning processes.
e) Utilities (e.g., HVAC, purified water systems).
f) Computerized systems. (e.g., Manufacturing Execution Systems, (LIMS) laboratory information management system).
g) Environmental monitoring.
3. Validation Categories and Types
The validation schedule should be structured by categorizing the validation into different types:
3.1. Equipment Qualification
Installation Qualification (IQ): Ensures that equipment is installed correctly.
E.g., New equipment installations, equipment relocations.
Operational Qualification (OQ): Confirms that equipment operates according to design specifications.
E.g., Temperature, pressure, and speed verification.
Performance Qualification (PQ): Validates that equipment consistently produces the intended results.
E.g., Long-term production performance under normal operating conditions.
3.2. Process Validation
Initial Process Validation: Conducted when introducing new processes or products.
E.g., Validation of tablet compression processes.
Ongoing/Continued Process Validation: Performed periodically to confirm the continued performance of established processes.
E.g., Annual revalidation of key processes.
3.3. Cleaning Validation
Ensures that the cleaning procedures are effective and there is no cross-contamination between batches.
E.g., Cleaning validation for different product changeovers on shared equipment.
3.4. Analytical Method Validation
Verifies that analytical methods produce reliable, accurate, and repeatable results.
E.g., Validation of assay, dissolution, or impurity tests used in quality control.
3.5. Computerized System Validation
Ensures that computerized systems are functioning according to regulatory requirements and intended use.
E.g., Validation of Manufacturing Execution Systems (MES) or Laboratory Information Management Systems (LIMS).
3.6. Utility Validation
Validation of support systems like HVAC, water systems, and compressed gases.
Water System Validation: Periodic validation to ensure the quality of purified water and water for injection (WFI).
HVAC Validation: Validation of temperature, humidity, and air quality in controlled areas (cleanrooms).
3.7. Revalidation
Periodic revalidation for equipment, processes, and systems that have previously been validated.
Triggered by changes such as equipment modifications, facility changes, or deviations from quality performance.
4. Frequency of Validation Schedule
The schedule should outline the frequency of validation activities, such as:
Annual validation review for all critical systems and processes.
Periodic revalidation every 1–3 years depending on the system’s criticality (e.g., HVAC, water systems).
Ongoing process validation based on production trends and product performance.
Equipment revalidation based on use, wear, or after maintenance activities.
5. Risk-Based Approach
The validation schedule should adopt a risk-based approach, with high-risk processes or equipment validated more frequently. For example:
Critical equipment (e.g., sterile manufacturing equipment, autoclaves) may require more frequent validation or revalidation.
Less critical systems (e.g., non-critical utilities or auxiliary equipment) may be validated at longer intervals.
6. Documentation Requirements
For each validation activity in the schedule, the following documentation must be generated:
Validation Plan: Detailed outline of what will be validated, by whom, and when.
Protocols (IQ/OQ/PQ): Documented protocols describing validation steps and acceptance criteria.
Validation Report: Summary of results, deviations, and conclusions.
Change Control Documentation: Any changes to the validated state must go through change control procedures.
Revalidation Reports: If revalidation is triggered by changes or periodic reviews, documentation should include updated protocols and reports.
7. Training
All personnel involved in validation must receive training on validation protocols and procedures.
The validation schedule should include periodic retraining sessions to maintain personnel competency.
8. Deviation Management
The schedule should incorporate a system for managing deviations observed during validation:
Deviation reporting and investigation must occur if any protocol acceptance criteria are not met.
Corrective and Preventive Actions (CAPA) should be initiated and recorded as part of the
validation.
9. Validation Schedule Format
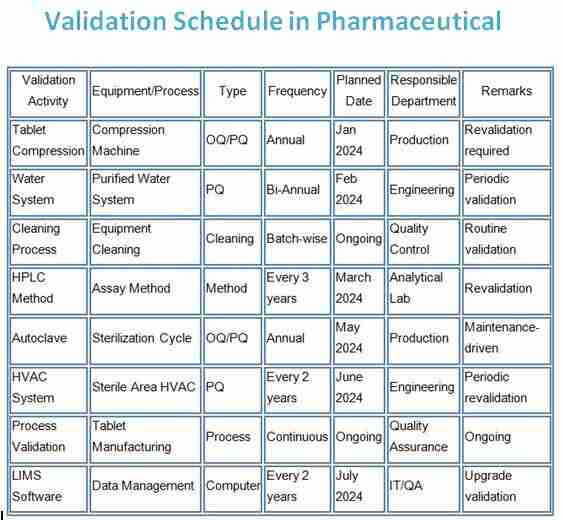
Below is an example of how a validation schedule can be presented in tabular form:
| Validation Activity | Equipment/Process | Type | Frequency | Planned Date | Responsible Department | Remarks |
| Tablet Compression | Compression Machine | OQ/PQ | Annual | Jan 2024 | Production | Revalidation required |
| Water System | Purified Water System | PQ | Bi-Annual | Feb 2024 | Engineering | Periodic validation |
| Cleaning Process | Equipment Cleaning | Cleaning | Batch-wise | Ongoing | Quality Control | Routine validation |
| HPLC Method | Assay Method | Method | Every 3 years | March 2024 | Analytical Lab | Revalidation |
| Autoclave | Sterilization Cycle | OQ/PQ | Annual | May 2024 | Production | Maintenance-driven |
| HVAC System | Sterile Area HVAC | PQ | Every 2 years | June 2024 | Engineering | Periodic revalidation |
| Process Validation | Tablet Manufacturing | Process | Continuous | Ongoing | Quality Assurance | Ongoing |
| LIMS Software | Data Management | Computer | Every 2 years | July 2024 | IT/QA | Upgrade validation |
10. Review and Updates
The validation schedule should be reviewed regularly (e.g., annually) and updated based on process changes, regulatory updates, and equipment modifications.
A review team, including representatives from Quality Assurance, Production, Engineering, and Regulatory Affairs, should oversee the schedule update process.
Conclusion
This Validation Schedule ensures that a pharmaceutical facility maintains a state of control over its processes, equipment, and systems, guaranteeing compliance with GMP and regulatory requirements while ensuring product safety and quality.
Frequently Asked Questions (FAQs)
What is a validation schedule, and why is it important?
A validation schedule is a planned timeline that outlines when specific equipment processes, or systems within a pharmaceutical or manufacturing facility will undergo validation or revalidation. It ensures that all critical operations comply with regulatory requirements and are consistently producing products that meet quality standards.
Importance:
1) Ensures equipment and processes perform as intended.
2) Helps maintain product quality and patient safety.
3) Aligns with regulatory requirements, avoiding compliance issues.
4) Facilitates timely identification of any deviations or failures that may affect the product
How is the frequency of validation or revalidation determined in a validation schedule?
The frequency of validation or revalidation is based on several factors, including:
Regulatory guidelines: Different authorities, like the FDA, EMA, and WHO, provide recommendations on how often validation should occur.
Risk assessment: Equipment and processes with higher criticality or potential impact on product quality may require more frequent validation.
Changes to systems: If there are changes in equipment, processes, or raw materials, revalidation is needed to ensure continued compliance.
Historical performance: Systems with a history of consistent performance may need less frequent revalidation, while those with frequent deviations may need more frequent validation.

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



