Learn how to write a (Standard Operating procedure) SOP for training and evaluation of the employees in the pharmaceuticals.
1. PURPOSE
To describe the standard operating procedure of the training system of introducing a newly appointed employee & the existing employee of the pharmaceuticals according to their nature & environment of the job
2. SCOPE
This SOP is applicable for all training and evaluation of all employees working at Pharma Boss Pharmaceuticals Limited.
3. RELATED DOCUMENTS
All SOP and documents associated with training.
4. PRECAUTIONS
Nil
5. RESPONSIBILITIES
5.1 Sr. Executive Admin is responsible for arranging an ‘Orientation Program’ for the employee and follow-up of this program.
5.2 Head of Quality Assurance or his nominee is responsible for briefing on different aspects of GMP/cGMP to the employee.
5.3 Respective Departmental Head or Manager is responsible for briefing the new employee about his/her job responsibility and to interaction with other departments. He or she also responsible to select the general SOPs for orientation program.
5.4 Departmental heads are responsible to assess the training needs and prepare YTS. He /she is also responsible to provide training as per YTS and on any new & revised SOP.
5.5 Head of Quality Assurance or his /her nominee is responsible to circulate the yearly training schedule and it’s follow-up.
5.6 Other departmental heads are responsible for briefing the new employee about the key functions of his/her department and interaction of them with respective department. 5.7 All trainees are responsible to attend the training on time and fill-up the training record.
6. ACCOUNTABILITY
Head of the Quality Assurance Department: Responsible for implementation, arrange and evaluation of training programmes.
7. PROCEDURE
7.1 Orientation Program for newly employed employee.
7.1.1 Arrange an ‘Orientation Program’ for the newly appointed employee in consultation with
Quality Compliance & the respective departmental head of the employee for minimum 3 to 7 days.
7.1.2 Brief the employee on the organizational history, policy management, administration, and site rules, during orientation.
7.1.3 Provide guidelines on the following topics according to orientation program:
- Basic GMP
- Hygiene and Health requirements
- Safety guides
- Job responsibility & some general SOPs (e.g. Dress change procedure, Change control, SOP for SOP)
7.1.4 The employee reports to each department for few hours and brief him/ her about the key functions of that department and interactions of this department with respective department.
7.1.5 Brief the employee about his/ her job description and interaction with other departments.
7.1.6 Assess the primary needs and provide training through orientation program & fill up the training record according to SOP/QA/009.
7.1.7 After completion of training, evaluate the trainee by an exam & publish the evaluation result according to Appendix B.
7.1.8 Assess the further needs for training and inform the requirement for yearly training program.
7.2 Training Program of Existing Employee
7.2.1 Assess the training needs for existing employees & inform it to the Quality Assurance Department.
7.2.2 Prepare a training schedule by the respective department & send the schedule to Quality Assurance.
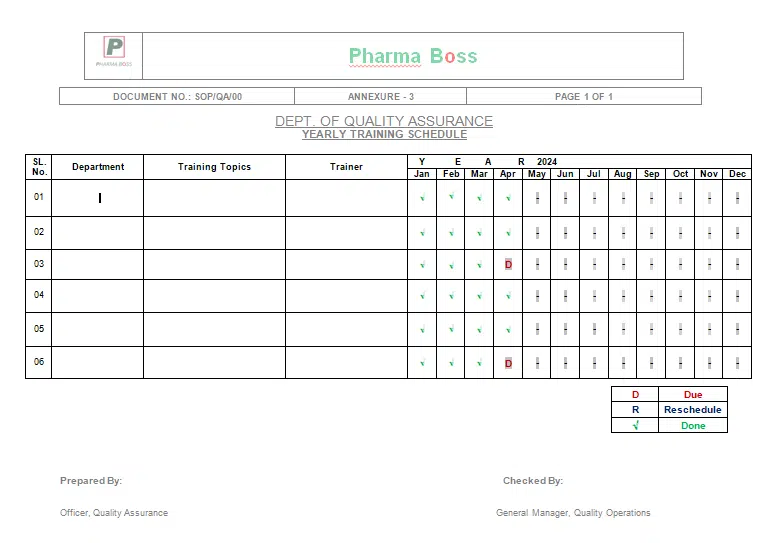
7.2.3 Circulate the Yearly Training Schedule. Which includes the followings but not limited to
- General GMP/cGMP / GLP/ GDP training.
- Hygiene & safety training.
- Departmental SOP training etc.
7.2.4 Arrange the training program according to the schedule.
7.2.5 Arrange any training by any department (if necessary) beyond the YTS (Appendix C) with prior information to Quality Assurance Department.
7.2.6 Record the training according to the SOP for training records of the employee.
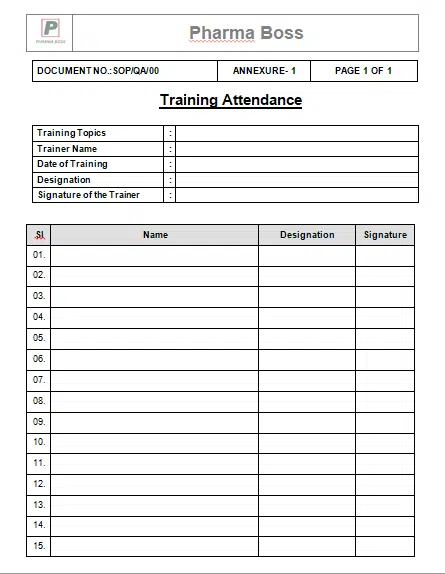
7.3 Take signature of the trainer & all trainees in attendance sheet (Appendix A) and send the
attendance sheet to Quality Assurance for record keeping.
Read Also: SOP for Annual Product Quality Reviews (APQR)
8. ABBREVIATIONS
8.1 SOP: Standard Operating Procedure
8.2 GMP: Good Manufacturing Practice
8.3 cGMP: Current Good Manufacturing Practice
8.4 GLP: Good Laboratory Practice
8.5 GDP: Good Documentation Practice
8.6 YTS: Yearly Training Schedule
9. ANNEXURES
9.1 Annexure-1: Training Attendance
9.2 Annexure-2: Evaluation Result Sheet
9.3 Annexure-3: Yearly Training Schedule
Annexure-1: Training Attendance
Annexure-3: Yearly Training Schedule

Abdus Sobhan Salim is professional experienced pharmacist in pharmaceuticals, author and founder of pharmabossbd.com, the first Bangladeshi pharmaceutical blogger since 2019.



